TMS Treatment FAQs
TMS Institute of Arizona offers the most advanced form of transcranial magnetic stimulation, MagVenture TMS Therapy. Those struggling with depression, anxiety, PTSD, insomnia, who have a major depressive incident, and other situations may benefit from this non-invasive treatment with no side effects and no risks associated with medications.
Is the TMS treatment for you? Call 480-448-2916 to schedule a detailed evaluation.
Video: What is TMS Therapy?
Video: Meet Richard, TMS Video Testimonial
Video: Meet Bari, TMS Video Testimonial
Video: TMS Q&A
Video: TMS Therapy and Depression
Video: Oliver’s Story
TMS is FDA-approved to treat depression, bipolar depression, obsessive-compulsive disorder (OCD), and smoking cessation. However, it has commonly been used “off-label” to treat a wide variety of symptoms and psychiatric and neurological conditions. We use evidenced-based research and protocols to treat conditions that have been shown to have significant improvement with a course of TMS therapy. These conditions include generalized anxiety disorder, panic disorder, post-traumatic stress disorder (PTSD), peri- and post-partum depression, addiction, autism spectrum disorder, insomnia and other sleep disorders, eating disorders, chronic pain, fibromyalgia, headaches, tinnitus, tremors, and cognitive disorders such as Alzheimer’s disease.
There is a risk that TMS may not work for you, just like with any other treatment. If TMS therapy does not provide any benefit, we advise discussing further treatment options with your psychiatrist and/or regular treatment clinician such as your primary care provider.
TMS is a durable treatment for depression with sustained responder rates of 65% up to one year after a successful induction course treatment. All known antidepressant treatments require a maintenance regimen to sustain positive results. TMS also follows this general rule. After your initial TMS series, you will be advised to monitor symptoms closely with your primary psychiatrist and therapist to evaluate for any worsening mood. If you and your providers determine that maintenance TMS is warranted, we will work with you to create a maintenance schedule that meets your needs. Yet, not all individuals that undergo TMS will need maintenance sessions. Under insurance coverage, if there is at least 50% improvement in depression symptoms after the initial course of TMS and then 6 months after completion there is regression of symptoms then insurance may approve a subsequent full course of TMS therapy. Also, TES therapy can be considered as well to aid in maintaining improvements gained with TMS therapy, which is a therapy performed at home.
Clinicians will carefully review your treatment regimen and work with you to help optimize your treatment while reducing any risks associated with psychiatric medications. In general, you can continue to take your regular medications during your TMS series. However, some medications may need to be stopped or reduced prior to initiating TMS to avoid the risk of a seizure. This will be discussed during your initial intake consultation visit.
The first studies using daily left prefrontal TMS for depression date back to the mid-1990’s at the National Institutes of Health (NIH) in Washington, DC. The technology has been investigated with millions of patients in the US and abroad. There are several meta-analyses that summarize the outcomes. In most cases, these “pooled analyses” have found TMS to be more beneficial than placebos in relieving depressive symptoms in treatment-resistant patients who failed more than one antidepressant drug therapy in their current depressive episode.
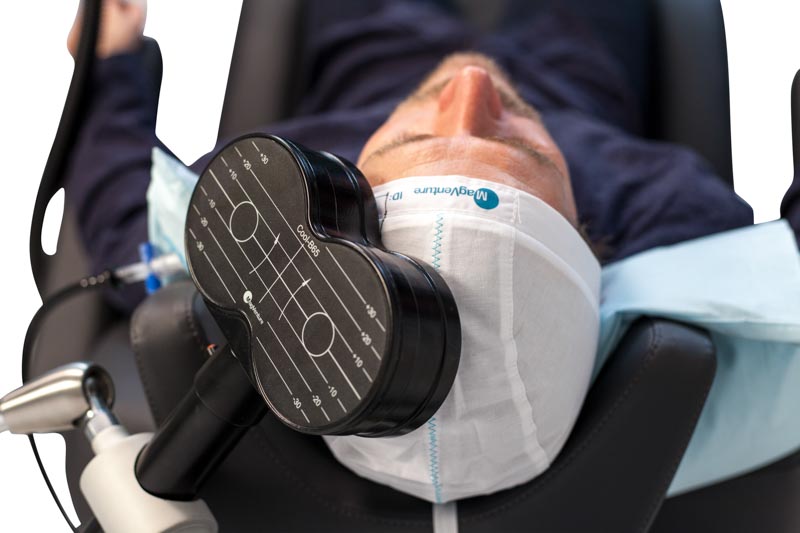
We are one of very few TMS centers in Arizona where the physician specifically performs the mapping procedure. During this session, the physician will take measurements of your head, perform the mapping, and determine the motor threshold. Mapping involves finding the specific target of stimulation, which is then mapped and marked on a cap that you wear for each treatment session. Next, the motor threshold determination is performed by pinpointing the minimum magnetic energy needed to stimulate your brain. This is performed by stimulating the left side of the brain over the area that controls your right hand. The stimulation will cause your right hand / finger / thumb to twitch. Now, the physician can determine the amount of stimulation to use in your case during the treatment sessions.
TMS has been well-studied in trials using a daily treatment model and, as such, we do not know if the effects will differ if patients cannot commit to a daily regimen. It is also important to know that most insurance companies provide authorization to complete the treatment sessions in a fixed time period. Our clinicians are experienced with this technology and will work with you to optimize a schedule that best fits your clinical and personal needs.
The TMS series of treatments varies per the protocol and indication, but the most commonly used protocol for depression consists of 5 treatments per week for 36 sessions. During the treatment session, patients sit comfortably in a chair similar to a dental chair with the coil resting lightly on their head. The device delivers focused magnetic pulses to stimulate the specific region of the brain being targeted. You can drive yourself to and from the center and immediately get back to work or school afterward. You are awake during the session and it does not require any sedation or pre-medication.













