Addiction and Smoking Cessation
Addiction (substance use disorder) is a global epidemic and can encompass addiction to nicotine, alcohol, marijuana, opioids, cocaine, methamphetamine, amphetamine, and even gambling. Substance use disorder (SUD) is a chronic disease that has the potential for both recurrence and recovery. Call 480-448-2916 to schedule a detailed evaluation.
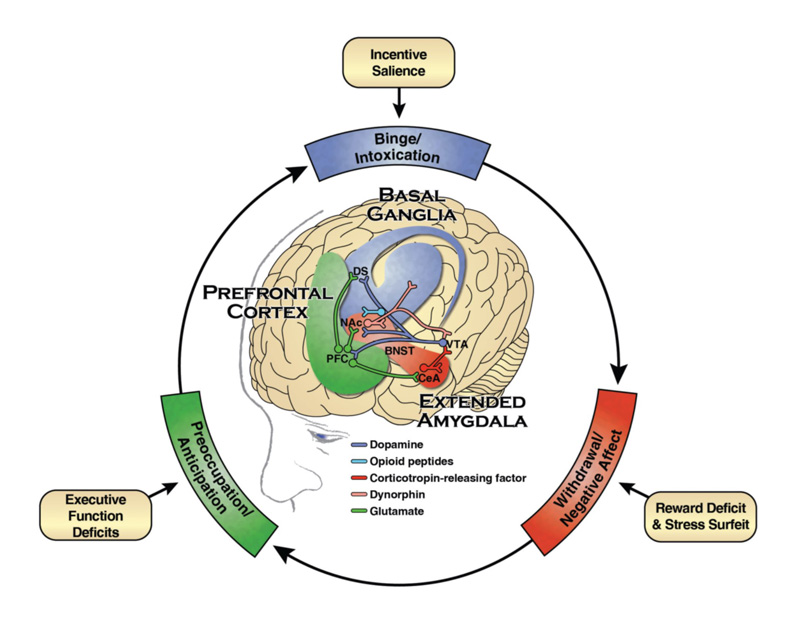
Substance use disrupts three primary regions of the brain: the basal ganglia, amygdala, and prefrontal cortex. Once disrupted, substance-seeking behavior is triggered. This leads to decreased brain sensitivity to pleasure or reward and reduced executive functioning ability to control impulses, actions, and emotions. These disruptions can continue to affect behavior even after an individual stops using substances.
TMS for Patients with Addiction + Smoking Cessation
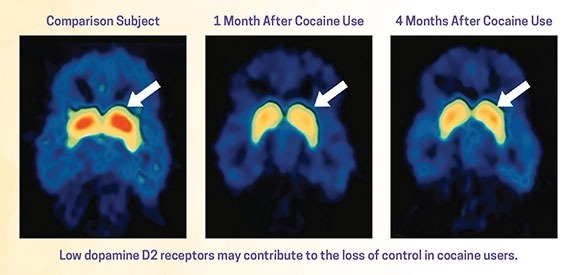
TMS for this off-label use requires applying high-frequency stimulation, which has been shown to reduce cravings post-rTMS treatment. MagVenture’s pivotal clinical study published in 2016 found a response rate of 69% for cocaine users that resulted in long-lasting abstinence for almost three years. This can be compared to a follow-up observational study published in 2020 which showed only a 19% success rate for those who received the standard care for addiction not involving TMS.
Smoking cessation has been shown to be successfully achieved using TMS therapy as well.
How TMS for Addiction + Smoking Cessation Works
TMS treatment for addiction and smoking cessation are similar, with addiction requiring a protocol of 17-minute sessions for 34 sessions. This treatment is not currently covered by insurance.
TMS can be used to treat several types of addiction, including:
- Alcohol / Cocaine/ Stimulants
- Dependence Cannabis / Heroin / Methamphetamine Dependence
- Smoking Cessation
- Nicotine Dependence
Off-Label Considerations
TMS may be helpful as an off-label treatment. Many clinical studies have been performed that support such treatments, but TMS is not yet approved by the FDA to treat disorders beyond depression and OCD. For off-label treatments, the machines are set differently in order to trigger appropriate and beneficial responses in the brain. It is a safe and effective alternative to medications that does not use anesthesia or have long-term side effects. To learn if you’re a candidate for TMS treatment, call the TMS Institute to schedule a detailed evaluation.

Call Our New Patient Coordinator for a Free Consultation.
Our New Patient Coordinator is here to answer all your questions so you can make the most informed decision.
She can explain our treatment options and protocols, fees, insurance, and more. She can also schedule you for a consultation with one of our doctors.
From there, we can determine if you may benefit from MeRT or TMS treatment, and you can decide if you would like to move forward.
We hope you will consider getting more information.