
Many people are suffering from depression, and it can be frustrating. The Sequenced Treatment Alternatives to Relieve Depression (STAR-D) trial shed light on this very issue, highlighting the limitations of traditional antidepressant approaches. But there’s a way forward.
Today, we will tackle the STAR-D Trial, a study that opened treatment pathways for depression years ago. We’ll also discuss the limitations of the STAR-D Trial and introduce a promising therapy gaining ground: Transcranial Magnetic Stimulation (TMS). Could TMS be the answer for those who haven’t found relief with traditional methods?
The STAR-D Trial
The STAR-D trial was a major study designed to assess the effectiveness of different treatments for depression. The aim was to find effective options in regular doctor’s offices (primary care) and specialist clinics.
Key Features
The trial has four levels, each with a different medication or combination from which the participants can choose. Each stage’s goal was to see if the treatment worked to help the participants feel better. Then, they would enter the next level with two options if they did not see any improvements. They can choose another treatment or add one.
One of the best parts of this trial is that it reflected real-world practice by allowing participants to choose their treatment plan preference. It was considered the first large-scale study to examine different approaches to treating depression.
Scope:
The study cost $35 million and involved over 4,000 participants diagnosed with major depressive disorder (MDD). The participants ranged in age from 18 to 75 and came from various ethnic and socioeconomic backgrounds.
They came from different clinics across the United States where they were already seeking treatment for depression. The goal of STAR-D was to include as many as possible to make the result applicable widely. However, there were some exceptions for people who couldn’t tolerate previous treatments, had certain medical conditions, or needed treatments outside of the specific options offered in the study.
Participations:
Not all people they approached for this study joined. There are over 1,000 people excluded. Reasons could be they did not meet the criteria for depression severity, or they just chose not to participate at all.
Also, the participants decreased in numbers throughout the different treatment stages (levels 2-4) as some people achieved remission (no longer depressed) and others dropped out for various reasons.
The STAR-D Treatment Journey and Monitoring
STAR-D worked by doing the trial step-by-step. It has different levels with different approaches.
Level 1: Starting Point
Patients were given Citalopram (SSRI) medication, which is known for being safe, easy to take, and with minimal withdrawal symptoms. The goal of this medication was to achieve remission, which means making each person symptom-free.
The same medication will continue to be applied to those who show improvement. But if no remission or any side effects are showing, they will be moved to the next level.
Level 2: Options for Continued Treatment
Here, the participants were given a chance to choose between switching medications or just adding additional medication (augmentation) to their existing citalopram.
For the switch option, they have the following:
- Sertraline (SSRI) – similar to citalopram but offers a comparison.
- Bupropion – works on different brain chemicals than SSRIs.
- Venlafaxine – affects two neurotransmitters at once for a broader impact.
Then, for the add-on options:
- Bupropion (non-SSRI antidepressant) – for a different approach if citalopram wasn’t enough.
- Buspirone (not an antidepressant) – helps existing medication work better.
- Cognitive psychotherapy – talk therapy as an alternative or alongside medication.
Their goal here was also to achieve remission. Likewise, participants who improved will stay on the current medication. Others will then move to level 3.
Level 3: Trying Different Mechanisms
People here can also choose between switching medications or adding something on.
For the switch options, they can opt for the following:
- Mirtazapine – a different type of antidepressant for a new approach.
- Nortriptyline (tricyclic antidepressant) – works differently than SSRIs used earlier.
For the add-on option, they can choose:
- Lithium (mood stabilizer) – helps regulate mood swings, potentially boosting antidepressant effects.
- Triiodothyronine (thyroid medication) – can sometimes improve response to antidepressants.
The aim was still to achieve remission. Successful participants continue treatment, while those who show no improvement will be moved to the final stage.
Level 4: Treatment-Resistant Cases
Changes occurred here. All the participants stopped their medications. They are now randomly assigned one of two options: tranylcypromine (MAOI), an antidepressant for severe cases, or the combination of Venlafaxine and Mirtazapine.
This last level aimed to find an effective treatment for difficult cases.
Alongside this, they implemented measurement-based care. They check the participants for symptoms and side effects using straightforward tools at their every visit. Also had a treatment guide that gives the right medication adjustments to lower side effects for a safe and maximization of the effectivity.
They used self-rating tools for the symptoms and side effects. These tools are easy and good for everyday use in clinics, allowing patients to report their experiences easily and accurately. Doctors can then use the reports to make treatment decisions.
STAR-D Trial Outcomes
Here’s a breakdown of how well each treatment worked:
Level 1 Result
Most people here are taking citalopram for medication. Using different measurement tools, it was found that 27-33% of people got better and reached remission. It took 47 days to see improvement. Race, job status, and education affected how well this treatment worked.
Level 2 Result
Switching Medications
Around 21-25% of people got better with each medication at this level. This did not reveal a significant difference between these medications in terms of effectiveness. Keep in mind that the doctor considers patients’ preferences when choosing a new medication.
Adding Medications
Here, they decided to add medication rather than switch. Only 30-39% of people got better with either additional medication. It was also found Bupropion tends to cause lower side effects than buspirone.
Cognitive Therapy
Aside from switching and adding, they also gave participants to try cognitive therapy. And around 31% of the participants improved with both options. Talk therapy was generally easier to tolerate than switching medications.
Level 3 Result
Switching Medications
Here, they tried different medications again. Mirtazapine or nortriptyline) had lower success rates (12-20% by HAM-D, 8-12% by QIDS-SR) compared to earlier levels. We can see that there is no major difference between these two medications.
Adding Medications
Adding medications like lithium or thyroid hormone (T3) to existing treatment had lower success rates (13-25%) than adding medications in level 2. T3 caused fewer side effects than lithium.
Level 4 Result
This is for highly resistant cases. At this level, a powerful medication combination or a medication called tranylcypromine had similar success rates (14-16%). It had fewer side effects and was easier to tolerate
Overall, about 67% of people improved with treatment across all levels. Also, earlier levels of treatment had higher success rates than later levels.
The graph below shows its effectiveness and failures from all the stages.
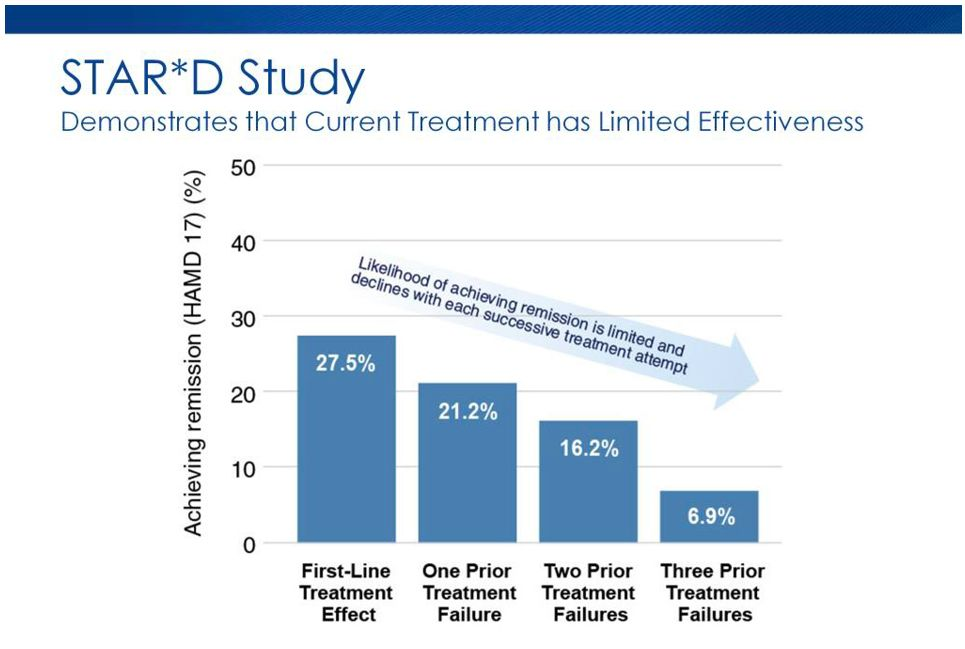
Graph Source: Bespoke Treatment
Limitations of the STAR-D Trial
As we can see, like any other study, STAR-D also has its limitations.
Choices and Comparisons
First, it made it challenging to compare the success of the treatment. If we recall, the trial gave the people the authority to choose between switching medications and adding medications (augmentation) if the initial treatment in level 1 did not work.
This is good because it shows real-world scenarios where patients can have their preferences. But, it became hard to compare the effectiveness because people who switched medications tend to be people with severe depression.
Therapy Representation
Since the study initially required citalopram for everyone in level 1, many of them did not choose talk therapy or cognitive therapy as an option in level 2. They may not want to pay extra or make an extra effort to visit another doctor for additional treatment.
Lack of Control Group
Another limitation is that it included another group receiving no medication (placebo) and a specific treatment plan to test whether the trial was effective.
Medication Dosages
Medication should be used at its full potential. But as we can see in the trial, it was only focusing on adjusting medication doses based on the reported symptom rather than giving the people a fixed dose. This may result in them giving lower dosages than what is really recommended.
Individual Results
Even though it reported the average success rate, it lacks or doesn’t tell us which treatment works best for each person. Additionally, the study was done many years ago, and many newer treatments were not included in the trial.
The Potential of TMS Therapy as a First-Line Treatment
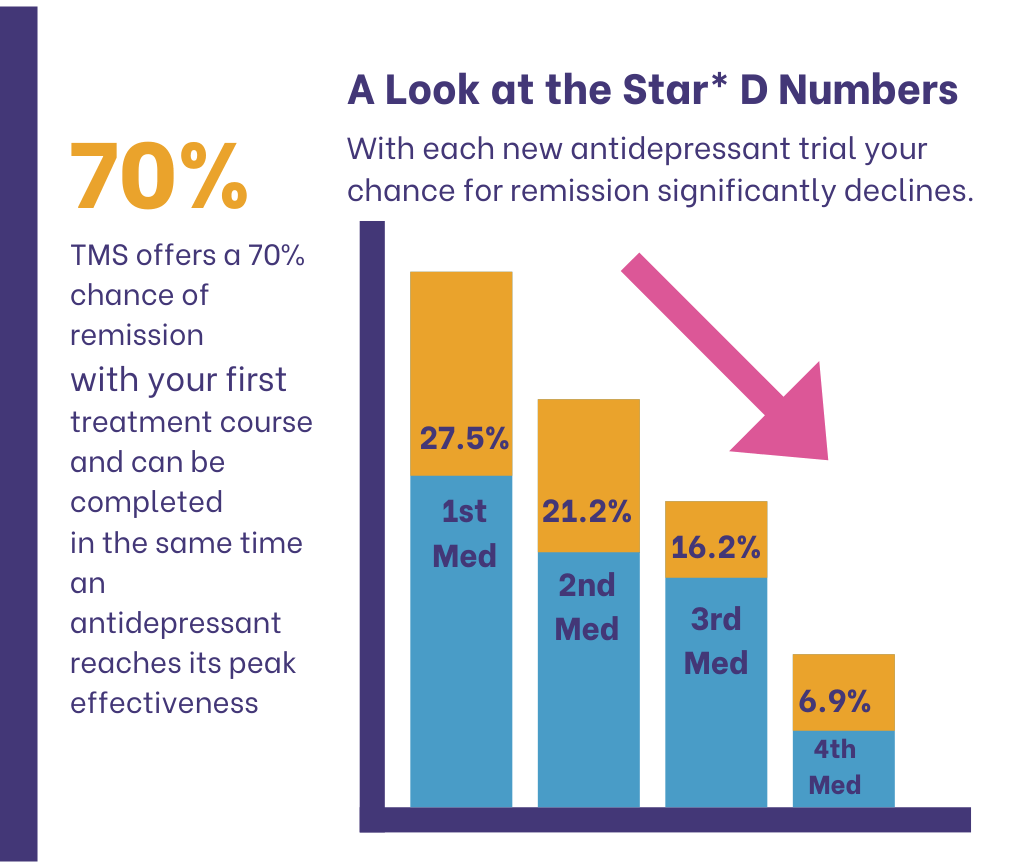
Though the STAR-D trial makes it easy for doctors to understand which depression treatment works, more options are available now. One is Transcranial Magnetic Stimulation (TMS) with a 70% chance of remission rate with patients on their first treatment course.
TMS is a treatment known for being noninvasive and 100% safe. The procedure uses magnetic fields to activate nerve cells in a specific brain area, potentially improving depression symptoms. Doctors like Dr. Richard A. Berudes from the American Psychiatric Association consider TMS therapy the first line of defense in treating moderate to severe depression.
He was first concerned that the 2010 APA guidelines would recognize the use of TMS. To his surprise, it was included along with other kinds of treatments. The guidelines emphasized the importance of ensuring patients can choose which treatment they want to try.
Although, electroconvulsive therapy (ECT), also a type of treatment for different types of mental health problems, is still needed, especially for treatment-resistant conditions. He suggested better prioritizing TMS and other non-invasive types whenever possible.
His support for TMS came from different research that supports its efficacy for moderate to severe depression, saying that it is just as effective, just like traditional medication and psychotherapy. It has no major side effects and has impressive lower discontinuation rates than medications.
Dr. Berudes APA article recommended TMS for patients who haven’t tried medication before (also called treatment-naive) and for those patients who tried their first course of antidepressants in the current episode but failed.
Another known Doctor, Dr. Ruchir Patel, encourages patients to try TMS, which is pain-free and FDA-approved. He, in fact, uses it to treat his patients with Major Depressive Disorder, OCD, Anxiety Disorder, PTSD, and other mental health conditions. Today, TMS delivers hope to anyone suffering mentally, making it the first line of defense in mental health.
How Can the TMS Institute of Arizona Help?
Having depression or any other mental problems is challenging. But at TMS Institute of Arizona, we offer hope. We have experienced doctors led by Dr. Ruchir Patel, who specializes in Transcranial Magnetic Stimulation (TMS) therapy, offering a non-invasive, pain-free, safe, and medication-free approach to treatment.
We know our patients have different needs, and we care for them. That’s why we offer a personalized approach with three primary treatment options: TMS therapy, MeRT therapy, and TES therapy.
We promise to work closely with you to assess your specific needs. And, we will recommend the treatment plan with the highest potential for success. We will be here for you through every step, ensuring you feel informed and supported throughout your journey to better mental well-being.
Sp, if you want to try TMS therapy or discuss options, contact us today at 480-448-2916 or fill out our contact form. We can help you find the path to healing.
References:
Questions and Answers about the NIMH Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Study — All Medication Levels. (2024). National Institute of Mental Health (NIMH). https://www.nimh.nih.gov/funding/clinical-research/practical/stard/allmedicationlevels
Gaynes, B., Warden, D., Trivedi, M., Wisniewski , S., Fava, M., & Rush, J. (2020). What Did STAR*D Teach Us? Results From a Large-Scale, Practical, Clinical Trial for Patients With Depression. Psychiatric Services. https://ps.psychiatryonline.org/doi/10.1176/ps.2009.60.11.1439
BERMUDES, R. (2022). TMS Should Be Considered as First-Line Treatment for Moderate to Severe Major Depressive Disorder. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2022.10.8.27
H Edmund Pigott. (2015). The STAR*D Trial: It is Time to Reexamine the Clinical Beliefs That Guide the Treatment of Major Depression. The Canadian Journal of Psychiatry/Canadian Journal of Psychiatry, 60(1), 9–13. https://doi.org/10.1177/070674371506000104












